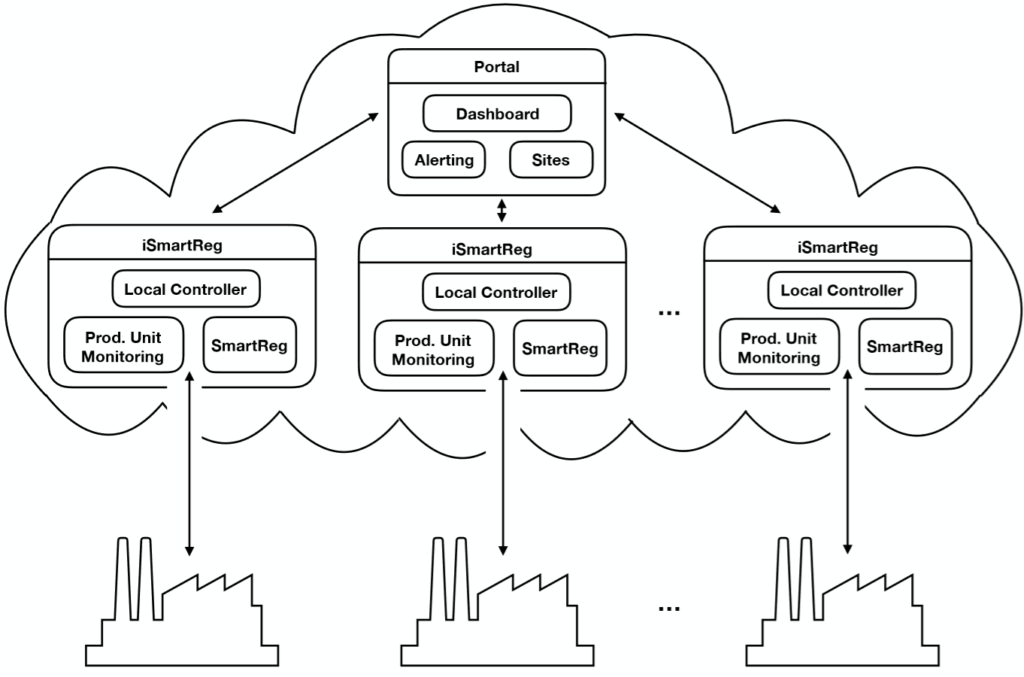
Level one
The first level relates to the data collected at the production stage: process and equipment parameters (for example incubators, centrifuges, microscopes, sensors, and scanners) and environmental parameters (such as pressure, temperature and relative humidity).