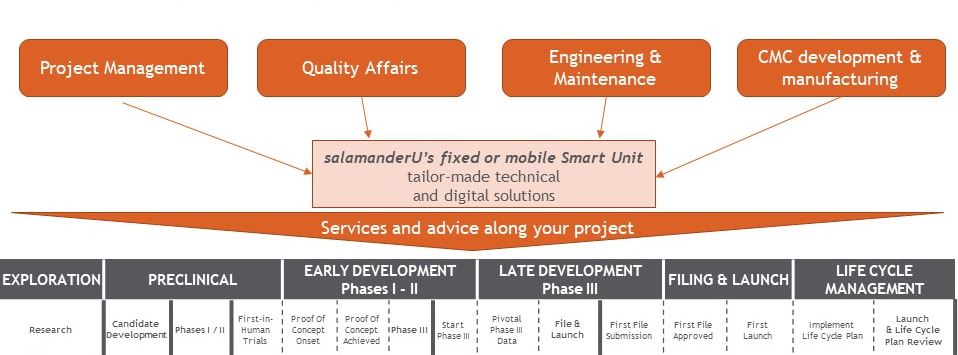
salamanderU accompanies you from your need to your regulatory inspection. This means we take into account all the aspects of your project, from analyzing your needs to making sure your installations and products comply with all applicable regulatory requirements.